There are five established biogenic amine neurotransmitters. Many aromatic amines have been reported to be potent mutagenic carcinogenic and hemotoxic agents.

What Are The Reactions For Amines And Amides Example
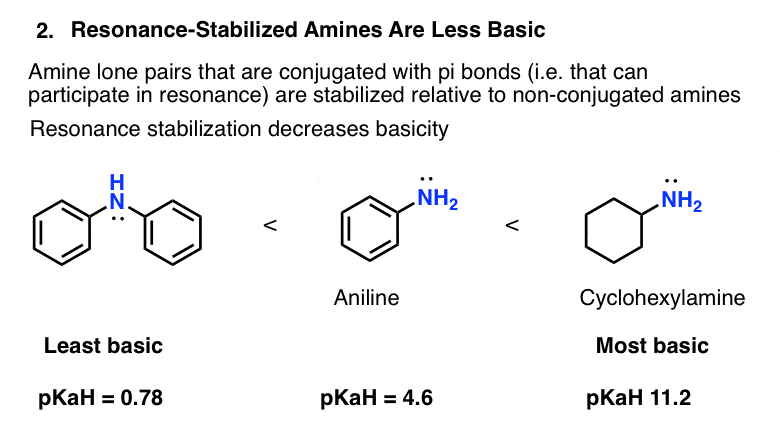
5 Key Basicity Trends Of Amines Master Organic Chemistry
Rule C 812 Primary Amines Groups Containing One Nitrogen Atom
Examples of direct uses of amines and their salts are as corrosion inhibitors in boilers and in lubricating oils morpholine as antioxidants for rubber and roofing asphalt diarylamines as stabilizers for cellulose nitrate explosives diphenylamine as protectants against damage from gamma radiation diarylamines as developers in photography aromatic diamines as flotation agents in mining as.
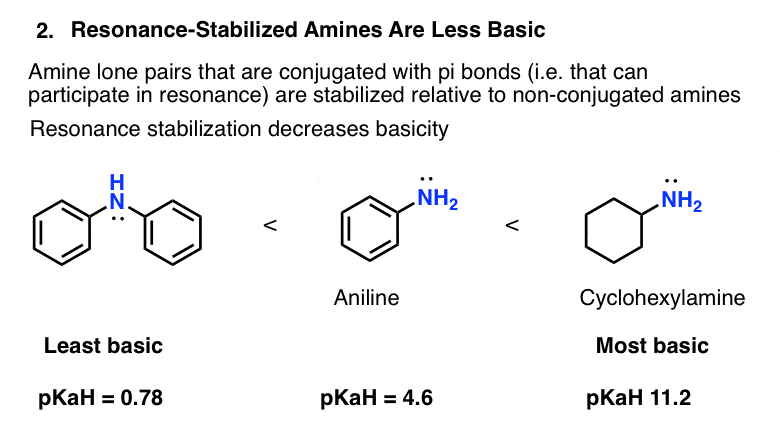
Examples of amines and their uses. Amines are weak bases that pick up a proton to form ammonium salts. Chapter 6 Amines and Amides 9 Examples. In terms of synthesis packaging release and degradation the amine neurotransmitters fall somewhere between the properties of the other small-molecule neurotransmitters and those of the.
Preparation of amines. Vitamins can be broadly delineated into 2 categories. Naming amines can be quite confusing because there are so many variations on the names.
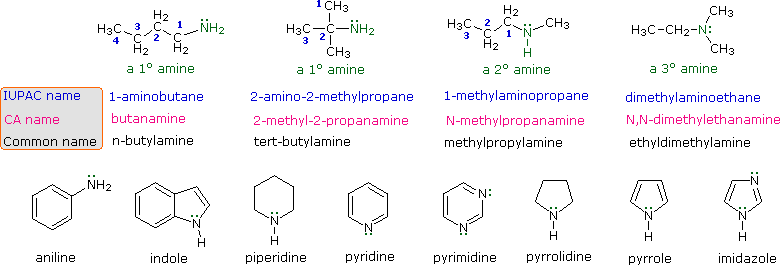
The nitrogen atom in an amine has a lone pair of electrons and three bonds to other atoms either carbon or hydrogen. Nomenclature of Amines Provide names for the following aromatic amines. Nomenclature of Amines Provide common names for the following 2 and 3 amines.
Norepinephrine is used in remedies for colds hay fever and asthma because it contracts the capillaries in the mucous membranes of the respiratory passages. N CH3 CH3 N CH3 CH2CH3 NH CH3 NH2 CH3. The sulfonamide of a primary amine is soluble in an aqueous base because it still possesses an acidic hydrogen on the nitrogen which can be lost to form a sodium salt.
Medicines based on amines such as Morphine and Demerol are commonly used as analgesics medicines that relieve pain. The importance of vital amines or as we know them vitamins. Examples for heterocyclic aromatic amines are pyrrole and pyrydine.
Among the amides of commercial importance are acetamide also called ethanamide CH 3 CONH 2 and dimethylformamide HCONCH 3 2 which are used as solvents the sulfa drugs and the nylons. The three catecholaminesdopamine norepinephrine noradrenaline and epinephrine adrenalineand histamine and serotonin see Figure 63. The oxidation of a tertiary amine leads to the formation of an amine oxide.
Pyrollidine and Pyridine via Wikicommons Public Domain. These salts are more soluble in water than the corresponding amines and this reaction can be used to dissolve otherwise insoluble amines in. An introduction to amines including the various types of amine primary secondary and tertiary and their physical properties.
Glutaraldehyde also has been used for conjugation of amines for example Vallejo et al. Urea or carbamide CONH 2 2 is a crystalline compound that is formed as the end product of the metabolism of protein and excreted in the urine of mammals. Reviewing some examples of their in a sentence should help you get its usage right.
CH3CCH2CH2CH2CHCH3 ONH2 OH NH2 10 Examples. A number of polycyclic aromatic amines are potent bladder carcinogens in animals as well as in humans. List of Uses of Amines.
However as these vitamins are water-soluble they are not efficiently stored in tissues. Amines such as Novocaine are commonly used as anesthetics. For 1 amines provide common andor IUPAC names where possible.
Morphine and Demerol are used as analgesics that are pain killers. Various nomenclatures are used to derive names for amines but all involve the class-identifying suffix ine as illustrated here for a few simple examples. The red one is their house.
Besides the amines that the human body is composed of amino acids humans have found a range of other uses for amines. Water-soluble vitamins are absorbed rapidly via the gastrointestinal tract and distributed widely in various body tissues. 166 used toluene-24-diisocyanate to couple 2- nitrophenylsulfenyl derivative of 5-3-hydroxypropyl-triazole to protein.
Novocaine is used as anaesthetic and Ephedra is a very common decongestant. Amines are molecules that contain carbon-nitrogen bonds. We use tetramethyl ammonium iodide for disinfecting drinking water.
Trimethylamine for example reacts with acid to form the trimethylammonium ion. Amines as bases. Instead of saying Thats the Murphy familys new dog you can say Thats their new dog While his and her demonstrate singular possession possession by one person their is reserved for two or more people or things.
In daily life activities amines are used for pest control and tanning of leather. The importance of the lone pair on the nitrogen in the reactions of amines as. They are used as intermediates in the manufacture of plastics drugs and carbamate pesticides.
Examples for aliphatic heterocyclic amines are Piperidine and Pyrollidine. 151 used glutaraldehyde as a cross-linker to couple 2-aminoparathion and reduced parathion to BSA. The commonest name at this level is methylamine and similarly the second compound drawn above is usually called ethylamine.
Amines are largely used in pharmaceutical industry. For example the simplest amine CH 3 NH 2 can be called methylamine methanamine or aminomethane. Although you can oxidize all amines only tertiary amines give easily isolated products.
Their preparation from halogenoalkanes haloalkanes or alkyl halides and from nitriles. Parkinsons disease is a result of a deficiency in another biogenic amine called dopamine.
Amine Reactive Crosslinker Chemistry Thermo Fisher Scientific Us
Amine Reactivity
Organic Nitrogen Compounds Iii Secondary And Tertiary Amines
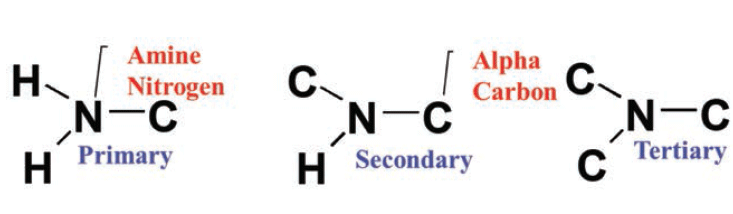
Difference Between Primary Secondary And Tertiary Amines Definition Basicity Examples Differences

Amine Reactions Of Amines Britannica

Amine Definition Structure Reactions Formula Video Lesson Transcript Study Com

Amine An Overview Sciencedirect Topics
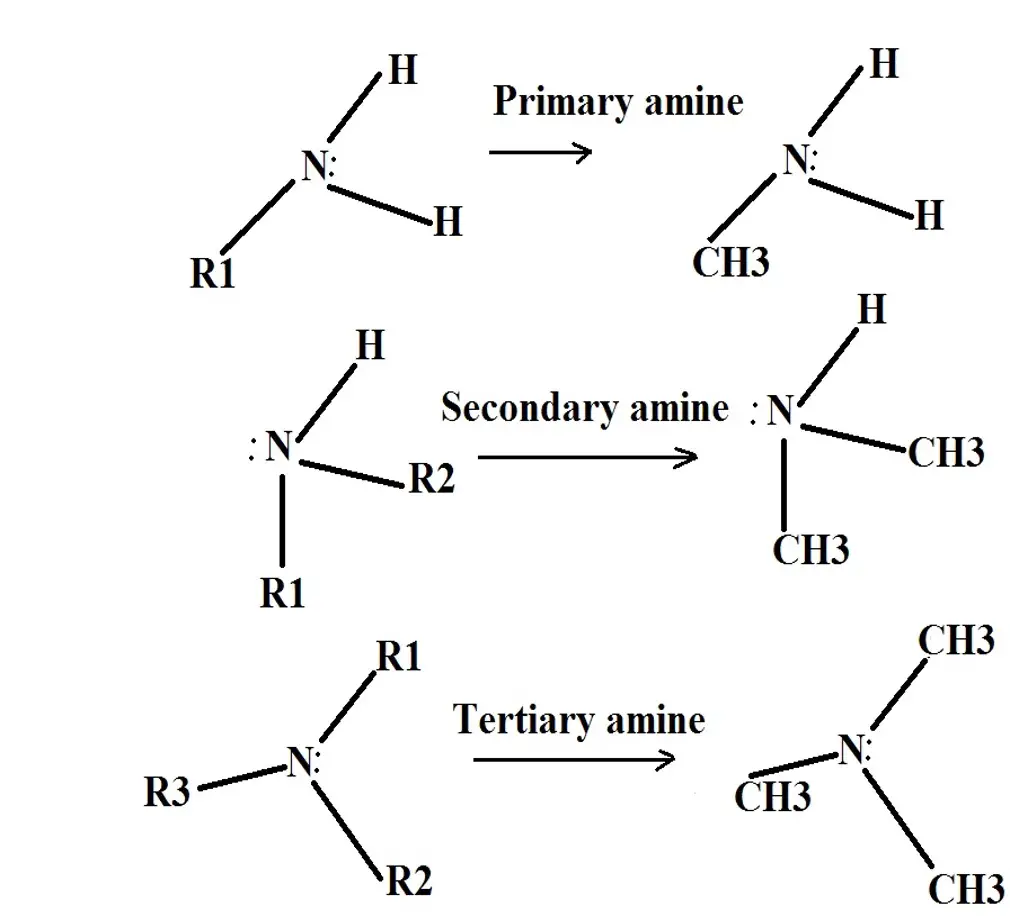
How To Identify And Classify Amines Examples And Characteristics
